Research Spotlight: Pinakin Gunvant Davey, OD, PhD, FAAO, FOWNS, FARVO

Dr. Pinakin Davey is a tenured Professor, in the College of Optometry and he also serves as the Director of Clinical Research at Center of Clinical Research at Western University of Health Sciences. He received his Bachelor of Science in Optometry from the Elite School of Optometry, India. He received his Ph.D. from Anglia Ruskin University in Cambridge, England, in the area corneal measurements and its influence on glaucoma. His post-doctoral research fellowship was on improving imaging techniques in glaucoma at the University of Louisville. He graduated with his Doctor of Optometry degree from Southern College of Optometry.
He is a fellow of the American Academy of Optometry and a Fellow of the Association for Research in Vision and Ophthalmology. Dr. Davey’s primary research interests are in the area of glaucoma and retinal physiology, particularly improving diagnostic abilities and visual outcomes in various disease states.
Dr. Davey has authored over 75 journal publications and book chapters and has given over 400 conferences and invited presentations both nationally and internationally.
Describe one of your current research projects.
All research that I do I very dear to me and this is usually the case with any scientist involved in active research. I would like to talk about accurate measurement of macular pigment AKA the yellow spot of the retina. The measurement of macular pigment optical density (MPOD) may have a lot more benefits than previously realized. MPOD is a biomarker that may aid in the evaluation and management of various disease states and healthy visual functions.
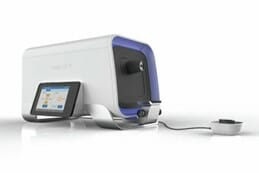
For example, the sub optimal levels of MPOD are biomarkers and risk factors for age related macular degeneration, diabetes and diabetic retinopathy, digital eye strain, poor visual performance and decreased cognitive function. The fascinating part of MPOD is that it is an “alterable risk factor/ biomarker”. So accurate measurement evaluating change in MPOD overtime is pivotal. Equally important is the convenience at which the MPOD can be measured. Whereas there are devices that can measure MPOD clinically, these are tabletop units that require space. They are not always convenient and may disrupt the patient flow in busy clinics (see Picture of desktop clinically available MPOD measuring technology). My recent work centered on assisting a group of engineers and scientists in miniaturizing the technology and improving its performance and reproducibility.

What do you want people to know about your research?
The research began by critically evaluating the software and the outcomes of the measurement. There were changes made to the software and to the way the MPOD was measured. Simple thresholds determining techniques commonly utilized in visual fields like devices were adapted to accurately estimate the MPOD. It takes a village to make a new device and although I was the principal investigator, The device will not be a reality without many unsung heroes. I am grateful to all that were involved, particularly the study participants that gave their valuable time.
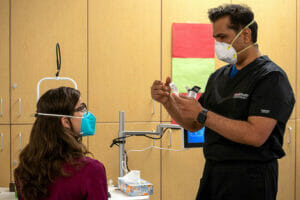
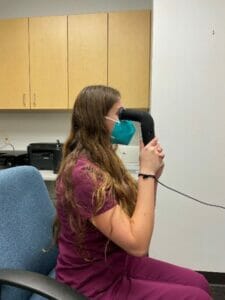
Early research involved looking at error scores and repeatability compared to predecessor devices that used similar principal to measure MPOD. You can see a student researcher examining MPOD on herself using an early prototype.
Subsequently I performed measurements on over 100 individuals to train the algorithm and independently test MPOD on participants that were novice to the testing using both the desktop and the handheld device.

How would you like people to use the results of your research?
The repeatability of the MPOD desktop devices has been evaluated previously by my lab and many other top laboratories. We find results that are consistent with previously published literature. Desktop units are repeatable with a correlation coefficient of 85%. The handheld units have greater a reproducibility of 90%. The devices had excellent agreement with each other. The scientific outcome for the study is that the handheld device has excellent repeatability and agreement with the predicate device. Due this study and the data collected it has led to the eventual launch of the unit commercially in April 2023, and will now be sold under the name Zx Pro by EyePromise LLC Chesterfield, MO, USA. The Zx Pro will provide doctors with a baseline of MPOD. Eyecare providers should measure MPOD regularly and the individuals that have lower MPOD should implement life-style changes with improved diet or take nutritional supplements with the carotenoids lutein and zeaxanthin to improve MPOD. This will lead to benefits to the eye and the body.
What inspires you most about your research?
Clinical research can influence and touch the lives of millions of patients and practicing physicians. My work has led to various clinically available technologies. When I see doctors using these in practice and loving the technology that made their life easier and can see patients benefiting from it, I derive pleasure in calling the technology “one of my babies”.